Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (10): 1596-1603.doi: 10.3969/j.issn.2095-4344.2017.10.020
Previous Articles Next Articles
Wallerian degeneration after peripheral nerve injury: research advance in nerve conduits
Chang Biao, Quan Qi, Sun Xun, Liu Ruo-xi, Wang Yu, Lu Shi-bi, Peng Jiang
- Orthopedic Institute of General Hospital of Chinese PLA, Beijing Key Lab of Regenerative Medicine, Key Laboratory of Trauma & War Injuries of Chinese PLA, Beijing 100853, China
-
Received:2016-11-26Online:2017-04-08Published:2017-05-08 -
Contact:Peng Jiang, M.D., Researcher, Orthopedic Institute of General Hospital of Chinese PLA, Beijing Key Lab of Regenerative Medicine, Key Laboratory of Trauma & War Injuries of Chinese PLA, Beijing 100853, China -
About author:Chang Biao, Studying for master’s degree, Orthopedic Institute of General Hospital of Chinese PLA, Beijing Key Lab of Regenerative Medicine, Key Laboratory of Trauma & War Injuries of Chinese PLA, Beijing 100853, China -
Supported by:the National Natural Science Foundation of China, No. 51073024, 51273021; the National Basic Research Program of China (973 Program), No. 2014CB542201, 2012CB518106; the Special Project of the “Thirteenth Five-year Plan” for Medicine Development of Chinese PLA, No. BWS13C029
CLC Number:
Cite this article
Chang Biao, Quan Qi, Sun Xun, Liu Ruo-xi, Wang Yu, Lu Shi-bi, Peng Jiang. Wallerian degeneration after peripheral nerve injury: research advance in nerve conduits[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(10): 1596-1603.
share this article
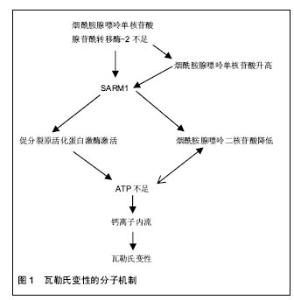
2.1 瓦勒氏变性分子机制 研究清楚瓦勒氏变性的分子机制对找到延迟周围神经瓦勒氏变性并促进其再生的靶点至关重要。1850年Augustus Waller教授最先报道在神经胞体远端切断神经后,损伤的远端轴突及部分近端轴突会发生崩解,髓鞘崩解脱失及细胞增生等一系列变化[15],这一现象被称为瓦勒氏变性,这是周围神经损伤及再生的研究基础。 延迟瓦勒氏变性(WLDS)蛋白变异大鼠的偶然发现,使科学家们认识到了瓦勒氏变性是一个主动的过 程[16],相似但又不同于凋亡[17]。野生型小鼠神经损伤后36 h左右发生变性,而WLDS够延迟瓦勒氏变性2周[18]。随着对WLDS功能的深入研究,烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2[2]、SARM1等一系列因子得以被发现[11]。这些因子之间形成多种信号通路,最终导致轴突内能量耗尽,导致钙离子大量内流[19]。正是钙离子的内流导致了损伤神经瓦勒氏变性的发生[6,14,19]。所以,瓦勒氏变性过程更加倾向于轴突内能量供应出现问题而导致。本部分主要描述瓦勒氏变性前的分子机制及分子间的关系(图1)。这些研究的发现可能为周围神经损伤提供了理想的治疗靶点。如何将这些重要的因子应用于周围神经修复呢,神经导管的研究为这一设想提供了可能性。 2.1.1 WLDS的功能 WLDS在很多物种及疾病模型之中都具有神经保护作用[16,20-23]。WLDS主要由N端Ube4b和C端的烟酰胺腺嘌呤单核苷酸腺苷酰转移酶1组成[24],两者对于WLDS在都很重要。烟酰胺腺嘌呤单核苷酸腺苷酰转移酶1是一个烟酰胺腺嘌呤二核苷酸合成酶,由于烟酰胺腺嘌呤二核苷酸对能量代谢很重要,所以被认为是最重要的作用成分[8,25-27]。Ube4b中的一部分发挥作用使WLDS定位于轴浆。最近一项研究通过基因调控WLDS的稳态来控制WLDS在轴突内的表达,证明了WLDS在轴浆中持续的表达发挥了其神经保护作用[4]。通过研究发现,WLDS实际上就是烟酰胺腺嘌呤单核苷酸腺苷酰转移酶1在轴浆中的异位高表达发挥神经保护作用。神经损伤后轴突降解被证明是可以延迟的。有趣的是,神经损伤后只有在4 h之内加入WLDS才能起到作用[4],这提示还有一些其他的分子在瓦勒氏变性过程中起重要作用。 2.1.2 烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2的功能及与烟酰胺腺嘌呤二核苷酸代谢的关系 由WLDS的神经保护功能可推断出烟酰胺腺嘌呤单核苷酸腺苷酰转移酶可能在瓦勒氏变性中起很大的作用。烟酰胺腺嘌呤单核苷酸腺苷酰转移酶有3种亚型。烟酰胺腺嘌呤单核苷酸腺苷酰转移酶1主要位于细胞核内,烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2主要位于胞浆和轴浆之中,烟酰胺腺嘌呤单核苷酸腺苷酰转移酶3主要位于线粒体 内[28]。虽然通过异位表达或过表达证明了烟酰胺腺嘌呤单核苷酸腺苷酰转移酶1和烟酰胺腺嘌呤单核苷酸腺苷酰转移酶3也能够抑制瓦勒氏变性[9,29],但通常是异位表达于轴突内才能发挥作用。烟酰胺腺嘌呤单核苷酸腺苷酰转移酶1、烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2、烟酰胺腺嘌呤单核苷酸腺苷酰转移酶3三种基因敲除小鼠模型的建立最终证明了在轴突中最重要的抑制瓦勒氏变性的因子是烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2[2-3,30-31],因为只有烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2敲除后会导致瓦勒氏变性的发生,而其他2种敲除后轴突不会发生降解。在果蝇中烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2的同源基因烟酰胺腺嘌呤单核苷酸腺苷酰转移酶也有同样的作用[32]。 烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2在促进烟酰胺腺嘌呤单核苷酸向烟酰胺腺嘌呤二核苷酸的转化上发挥了重要的作用,这就间接地提示了烟酰胺腺嘌呤二核苷酸可能在瓦勒氏变性过程中发挥很重要的作用[15-16]。烟酰胺腺嘌呤二核苷酸和烟酰胺腺嘌呤二核苷酸磷酸在氧化还原反应中作伪辅酶起传递电子的作用,尤其是在为各种重要的新陈代谢活动提供能量过程中很重要[33]。而且在氧化还原反应中,烟酰胺腺嘌呤二核苷酸,烟酰胺腺嘌呤二核苷酸磷酸和它们的还原状态烟酰胺腺嘌呤二核苷酸,烟酰胺腺嘌呤二核苷酸磷酸,能在不消耗的情况下互相转变。除此以外,烟酰胺腺嘌呤二核苷酸还是多种烟酰胺腺嘌呤二核苷酸消耗酶的底物,如SIRTs,PARPs,CD38。烟酰胺腺嘌呤二核苷酸和它的底物在很多重要生理活动中发挥作用,如生长、衰老等[33]。 烟酰胺腺嘌呤二核苷酸有3条生物合成通路,其中对于维持轴突中烟酰胺腺嘌呤二核苷酸水平最重要的是补救合成通路[5],而烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2正是在其中发挥了重要的作用。 有教授对轴突内烟酰胺腺嘌呤二核苷酸的水平做了相关检测。他们惊奇地发现,在表达WLDS的轴突内,烟酰胺腺嘌呤二核苷酸的水平与野生型的没有区别[10,34]。体外培养的背根神经节轴突损伤后4 h烟酰胺腺嘌呤二核苷酸水平开始降低,但是轴突保持完整。6 h才出现轴突降解的迹象,此时烟酰胺腺嘌呤二核苷酸已不能检测到[9]。长春新碱,一种能破坏微管的药物,也能制造出与这个类似的模型[9]。然而另一组研究发现烟酰胺腺嘌呤二核苷酸的降低能被更早的引出[35]。时间差异可能与体内、体外培养有关。 一些研究指出,烟酰胺腺嘌呤二核苷酸在延迟瓦勒氏变性过程中发挥很大的作用[9],因为WLDS能够阻止损伤轴突内烟酰胺腺嘌呤二核苷酸和ATP的降低。有些专家将外源性烟酰胺腺嘌呤二核苷酸的应用到损伤的轴突中,发现能够延迟瓦勒氏变性,但是必须在损伤后4 h内加入[4],但仍有一些专家持怀疑态度[36]。分歧的作用效果也可能与添加的浓度有很大的关系[5,9-10]。几种烟酰胺腺嘌呤二核苷酸的前体也具有神经保护作用,但是由于作用强度不同,作用的时间点有差别,而且一般比烟酰胺腺嘌呤二核苷酸的作用弱[5]。 烟酰胺腺嘌呤单核苷酸作为烟酰胺腺嘌呤二核苷酸的前体,近些年的研究显示其既具有保护神经的作用,也有促进神经发生瓦勒氏变性的作用[5,7,28]。促进轴突降解的机制主要与SARM1蛋白的激活有关,其保护作用主要与提高烟酰胺腺嘌呤二核苷酸水平有关[4-5]。 因为烟酰胺腺嘌呤二核苷酸在氧化还原反应中的传递电子的重要作用,烟酰胺腺嘌呤二核苷酸不足会阻碍ATP的产生,导致轴突运输受损及线粒体功能障碍。于此同时ATP不足会影响烟酰胺腺嘌呤二核苷酸合成过程(烟酰胺腺嘌呤单核苷酸+ATP=烟酰胺腺嘌呤二核苷酸+PPi),造成恶性循环,主流观点是烟酰胺腺嘌呤二核苷酸不足为始动因素。 现在研究认为神经损伤后轴突内烟酰胺腺嘌呤二核苷酸的不足主要还是由烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2的不足引起的。烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2的半衰期只有0.6 h左右[37],其从胞体中的高尔基体囊泡通过快速轴浆运输到达轴突,由于轴突连续性的终断,不能从胞体得到补充,很快降解完全,导致烟酰胺腺嘌呤二核苷酸合成受阻。烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2的半衰期虽然能够被多种因子影响[38-39],但作为治疗靶点的可能性不是很高。 2.1.3 SARM1 大鼠SARM1和同源物果蝇dSARM在促进瓦勒氏变性中起关键作用。在SARM1基因敲除的大鼠中瓦勒氏变性能够明显被延迟,效果和WLDS的保护作用几乎相同,表明烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2与SARM1可能位于同一条通路内[35]。SARM1激活后烟酰胺腺嘌呤二核苷酸和ATP会快速降低,而且SARM1促进烟酰胺腺嘌呤二核苷酸降低的速度比ATP快[35],这可表明烟酰胺腺嘌呤二核苷酸对ATP生成很重要,烟酰胺腺嘌呤二核苷酸降低是瓦勒氏变性的原因,而不是结果[35]。综上研究,作者认为SARM1通过降低烟酰胺腺嘌呤二核苷酸进而降低ATP导致轴突降解。 但是另一些研究显示SARM1的敲除不能阻止损伤轴突中烟酰胺腺嘌呤单核苷酸和烟酰胺腺嘌呤二核苷酸各自的升高和降低[40],这一结果明显和前述不同[35],这可能与浓度测定时标准化的腺苷酸池不固定有关。因为腺苷酸池包括ATP、ADP、AMP,其是动态变化的。 通过使用转基因小鼠,专家还发现了烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2和SARM1之间的关系[40]。烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2位于SARM1的上游,并且通过逆向推论,发现了SARM1位于促分裂原活化蛋白激酶(MAPK)通路的上游[41]。通过对促分裂原活化蛋白激酶通路中重要分子的抑制,发现促分裂原活化蛋白激酶通路激活后能够促使ATP消耗[41],最终导致瓦勒氏变性的发生。但是促分裂原活化蛋白激酶通路与烟酰胺腺嘌呤二核苷酸之间的关系尚未能证明。 在研究SARM1的过程中,专家发现了烟酰胺腺嘌呤单核苷酸促进瓦勒氏变性的作用[6-7]。相关研究发现了烟酰胺腺嘌呤单核苷酸作用于SARM1和钙离子内流的上游[6],因为利用烟酰胺腺嘌呤单核苷酸清除酶降低烟酰胺腺嘌呤单核苷酸水平后能够明显抑制瓦勒氏变性这一过程。作者认为烟酰胺腺嘌呤单核苷酸/烟酰胺腺嘌呤二核苷酸比例的改变可能对SARM1的激活起一定的作用,还需要进一步的研究。 2.1.4 轴突的能量供应 由于线粒体在有氧氧化功能方面具有非常重要的功能,线粒体曾被认为在瓦勒氏变性起始阶段发挥决定作用[42-45],但是这些模型均伴随轴突切断,这对线粒体功能会造成一定影响。最近的研究表明线粒体功能障碍不是瓦勒氏变性的起始因子,而是作为一个重要参与成分[6,14,28]。主要由于损伤后轴突内质网内钙离子释放传递至线粒体导致线粒体功能受影响进而释放钙离子[46]。已有相关研究证明囊泡快速轴浆运输的ATP供应主要来源于糖酵解过程,而烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2则正是来自于高尔基体囊泡的快速轴浆运输[2],而不是线粒体的有氧氧化过程[47],提示烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2在瓦勒氏变性中的重要作用。糖酵解在瓦勒氏变性中的作用需要更加受到重视。 2.1.5 钙离子内流 钙离子是导致瓦勒氏变性发生的关键因子[14]。轴突内ATP供应不足时,钠/钾交换器功能障碍,最终导致钙内流。钙离子内流分为两个不同的阶段。最初阶段的钙内流是由于线粒体的功能受干扰而产生,损伤的近端及远端均会发生,而且并不能被WLDS所阻断。第二阶段的钙内流发生在轴突瓦勒氏变性之前,钙离子由轴突逐渐播散到线粒体,进而线粒体也出现功能障碍。最重要的是,这一阶段的钙内流能够被WLDS所阻断[14]。大量钙离子内流后导致卡配因的激活[43],最终导致损伤的轴突发生瓦勒氏变性。 本部分主要回顾了瓦勒氏变性过程中一些重要的调控因子及它们之间的关系(图1),如烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2、烟酰胺腺嘌呤单核苷酸、烟酰胺腺嘌呤二核苷酸、钙离子。这些因子主要在神经损伤后4 h左右发生变化,通过研究它们的作用及关系可能为早期神经修复可能提供理想的靶点。 2.2 神经修复 通过之前的分析已经发现了几个可能在延迟及促进周围神经再生方面发挥重要作用的因子,但是如何才能将其运用到治疗周围神经损伤呢,这就需要多学科的交流。周围神经损伤修复的金标准是自体神经移植,但是这会对患者造成供体部位神经功能障碍,是一种拆东墙补西墙的做法。由于学科交流,材料学不断应用于周围神经再生,神经修复导管的应用使周围神经再生充满了前景。 2.2.1 神经结构 神经导管主要是仿生神经的基本结构。周围神经由许旺细胞组成的髓鞘包绕,其中有很多神经束,而且神经束内具有神经纤维,神经束膜也是由许旺细胞组成,其间结缔组织填充,这些成分共同组成了神经轴突复杂的三维空间结构。许旺细胞作为神经的支持细胞,在神经再生过程中至关重要。其增生过程中会分泌多种细胞因子,如脑源性神经生长因子,神经生长因子,这些因子均能促进神经的再生[48]。 2.2.2 神经导管 神经修复导管经历了一个很长的发展历程,从起初只是简单地模仿神经的管状结构,到应用各种促进神经修复的因子,再到现在开发更加符合神经复杂的三维空间神经修复导管复合各种因子,神经修复有了很大的进展[49]。 理想的神经修复导管应该具有以下优点:①防止瘢痕形成同时通透性好,营养因子和代谢废物能通过;②力学性能好,能起到支撑作用;③可降解;④生物相容性好。为了达到这些要求,多种材料被用于神经导管中,研究结果显示有一定优越性[50]。 神经导管的主要进展: 提升三维仿生结构:为了更好的模仿神经结构,构建促进神经再生的微环境,一些微型孔道被加入到空导管中,并且三维神经导管更加具备取向性特征——成线性与长轴平行,纤维充当细胞外基质,提供机械性能支撑,引导轴突生长的性能得到提升[51]。电纺丝技术在这方面有其独特的优势:①材料多孔,柔韧性好;②纤维成取向性,能促进许旺细胞增殖,迁移,促进神经纤维生长;③微米、纳米级别的纤维表面积大,能更好的促进许旺细胞迁移和轴突再生;④能复合神经营养因子,并缓慢释放[52]。 复合营养因子:空导管内增加结构特性是提升神经再生的一种方法,尤其是对于神经缺损。但是功能恢复良好的神经数量较低,这归因于一系列因素,包括细胞外基质形成不足,神经营养因子供应不足,许旺细胞数目不足,许旺细胞迁移和增生降低,还可能与远端营养作用降低有关。为了提升有功能的神经再生,应用了细胞外生长因子,神经生长因子。 "
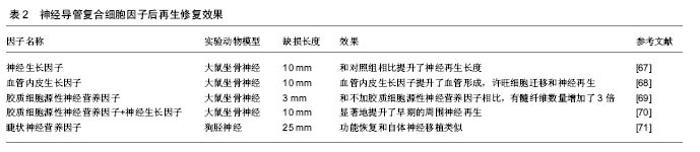
神经营养因子通过支持轴突生长,促进许旺细胞迁移和增生等来提升神经的功能恢复。神经营养因子主要属于3种不同的家族,神经营养因子,胶质细胞源性神经营养因子家族配体,和亲神经的细胞因子。神经营养因子包括神经营养因子3(NT-3),神经生长因子,脑源性神经营养因子;胶质细胞源性神经营养因子家族配体包括神经胶质细胞源性的神经营养因子,细胞因子包括睫状神经营养因子[53]。相关研究显示血管生成与神经形成具有协同作用。添加血管内皮生长因子能够明显提升血管生成,血管生成促进许旺细胞迁移,并提升神经再生[54-55]。这些神经营养因子单独或混合应用于神经导管中,提升了神经再生效果,而且联合应用取得的效果更好,因为周围神经包含不同的神经细胞亚群[52,56](表2)。单种神经生长因子释放效果不明显可能与起始阶段快速释放有关。通过优化神经营养因子释放动力学,如利用电纺丝缓释技术,也许能够克服它们的半衰期。 复合细胞:在神经再生期间,许旺细胞迁移和增生是神经修复的先决条件[57]。瓦勒氏变性后,许旺细胞迁移、增生形成bungner带,并大量分泌多种神经生长因子、层粘连蛋白等。然而,当缺损增大,许旺细胞迁移增生不能成线性排列并且许旺细胞数量不足,不能形成理想的再生环境。为了解决许旺细胞的这些问题,以细胞为基础的治疗方法被采用。当然,自体的许旺细胞复合入神经导管内被认为是现在以细胞为基础治疗的金标准。应用许旺细胞能产生神经营养因子,层粘连蛋白,表达细胞黏附分子并且能促进新生神经纤维形成髓鞘[58]。但是,自体许旺细胞的应用也有很多劣势:同自体神经移植一样,会导致供体部位功能残疾,需二次手术,对患者来说很痛苦;细胞培养时间长。所以,科学家将重点转移到干细胞。"
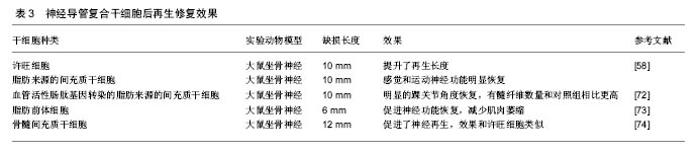
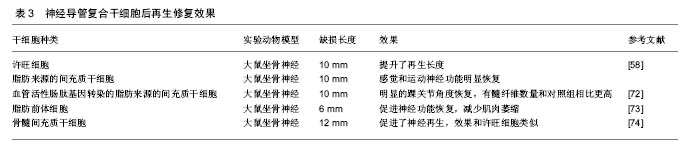
干细胞的应用以骨髓间充质干细胞和脂肪来源的间充质干细胞(ASCs)为主。骨髓间充质干细胞可以从患者骨髓中提取,脂肪来源的干细胞可以通过吸脂得到。这两类细胞有以下优点:数量多,易提取,繁殖快,成本低,副作用小,能分化为多种细胞系,尤其是神经系细胞——许旺细胞,并且这些细胞还能分泌多种神经生长因子59-61。这些细胞以应用于一些研究之中,结果显示能够促进神经的再生(表3)。近年诱导性多能干细胞研究进展很快,已成功被应用于修复神经损伤[59-62]。 国内进展:在神经再生领域国内专家在神经导管方面取得了很大的进展。南通大学顾晓松院士团队发明了壳聚糖固定加工技术,将壳聚糖与PGA 混合应用发挥了更好的神经修复效果,实现了对35 mm周围神经缺损的修复,其后通过在导管中添加蚕丝使再生的轴突取向性生长[63-64]。中山大学刘小林教授团队研发出了全球第二个去细胞移植物,已应用于临床,随访显示修复效果良好[65]。卢世璧院士团队也一直致力于对去细胞马尾神经的研究,在动物实验中已取得不错的效果。并且利用电纺丝技术构建出了多孔取向性神经导管。武汉理工大学戴红莲教授团队研发的发生纳米复合可降解神经导管,能对神经再生的纳米环境进行重塑,促进了神经生长因子的合成及表达,从而促进神经再生[66]。 综上可以看出,在神经修复的研究中,神经导管已取得较好的成果。但是神经导管促进神经再生的研究中主要关注于对神经结构及微环境中的一些营养因子,但还是没有将瓦勒氏变性的分子机制充分结合。如本文中第一部分讲的一些重要因子,如烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2,烟酰胺腺嘌呤单核苷酸等。虽然烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2的半衰期只有0.6 h,但有望通过与神经导管结合,将其应用。如利用电纺丝缓释的特点,达到烟酰胺腺嘌呤单核苷酸腺苷酰转移酶2长时间作用的目的。 WLDS大鼠是一个非常重要的实验对象,其周围神经损伤后瓦勒氏变性这一过程能够被延长至2周以上,由于延迟瓦勒氏变性主要是在损伤远端,促进神经再生主要在近端,制造WLDS大鼠神经缺损模型后能够更好地分析是否在延迟瓦勒氏变性的同时能够促进神经再生。 "
| [1]DeFrancesco-Lisowitz A, Lindborg JA, Niemi JP,et al. The neuroimmunology of degeneration and regeneration in the peripheral nervous system. Neuroscience. 2015;302: 174-203.[2]Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons.Endogenous Nmnat 2 is an essential survival factor for maintenance of healthy axons.PLoS biol.2010;8:e1000300 .[3]Gilley J, Adalbert R, Yu G,et al. Rescue of peripheral and CNS axon defects in mice lacking Nmnat 2. J Neurosci. 2013; 33(33): 13410-13424.[4]Wang JT, Medress ZA, Vargas ME,et al.Local axonal protection by WldS as revealed by conditional regulation of protein stability. Proc Natl Acad Sci U S A. 2015;112:10093- 10100.[5]Sasaki Y, Araki T,Milbrandt J.Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J Neurosci. 2006;26(33):8484-91..[6]Loreto A, Di Stefano M, Gering M,et al. Wallerian Degeneration Is Executed by an NMN-SARM1-Dependent Late Ca(2+) Influx but Only Modestly Influenced by Mitochondria. Cell Rep. 2015 22;13(11):2539-2552.[7]Di Stefano M, Nascimento-Ferreira I, Orsomando G, et al.A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration. Cell Death Differ. 2015;22(5):731-742.[8]Sasaki Y,Vohra BP, Lund FE,et al.Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J Neurosci. 2009;29(17):5525-5535.[9]Wang J, Zhai Q, Chen Y,et al. A local mechanism mediates NAD -dependent protection of axon degeneration. J Cell Biol. 2005;170(3):349-355.[10]Araki T, Sasaki Y,MilbrandtJ. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. Science. 2004;305(5686):1010- 1013.[11]Osterloh JM, Yang J, Rooney TM,et al.dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. Science. 2012;337(6093):481-484[12]Gerdts J, Summers DW, Sasaki Y, et al.Sarm1-mediated axon degeneration requires both SAM and TIR interactions.J Neurosci. 2013;33(33):13569-13580.[13]Kuan CY, Whitmarsh AJ, Yang DD, et al.A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci U S A. Proc Natl Acad Sci U S A. 2003;100(25):15184-15189.[14]Vargas ME,Yamagishi Y.Live Imaging of Calcium Dynamics during Axon Degeneration Reveals Two Functionally Distinct Phases of Calcium Influx. 2015;35:15026-15038.[15]Waller A. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Phil. Trans. R. Soc. Lond.1850;140:423-429.[16]Lunn ER,Perry VH,Brown MC,et al.Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur J Neurosci. 1989;1(1):27-33. [17]Deckwerth TL,Johnson EM Jr. Neurites can remain viable after destruction of the neuronal soma by programmed cell death (apoptosis). Developmental biology.1994;165:63-72.[18]Beirowski B, Adalbert R, Wagner D, et al.The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 2005;6:6.[19]Chang B, Quan Q, Lu S,et al. Molecular mechanisms in the initiation phase of Wallerian degeneration. Eur J Neurosci. 2016 ;44(4):2040-2048.[20]O'Donnell KC,Vargas ME,Sagasti A.WldS and PGC-1alpha regulate mitochondrial transport and oxidation state after axonal injury. J Neurosci. 2013 ;33(37):14778-14790.[21]Avery MA, Rooney TM, Pandya JD, et al.WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering.Current biology. Curr Biol. 2012 10;22(7):596-600.[22]Beirowski B, Babetto E, Coleman MP,et al.The WldS gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur J Neurosci. 2008;28(6):1166-1179.[23]Wang MS, Davis AA, Culver DG,et al.WldS mice are resistant to paclitaxel (taxol) neuropathy. Annals Neurology.2002; 52: 442-447.[24]Laser H, Conforti L, Morreale G, et al.The slow Wallerian degeneration protein, WldS, binds directly to VCP/p97 and partially redistributes it within the nucleus. Molecular biology of the cell. Mol Biol Cell. 2006;17(3):1075-1084.[25]Babetto E, Beirowski B, Janeckova L,et al.Targeting Nmnat 1 to axons and synapses transforms its neuroprotective potency in vivo. J Neurosci. 2010;30(40):13291-1304.[26]Sasaki Y, Vohra BP, Baloh RH,et alTransgenic mice expressing the Nmnat 1 protein manifest robust delay in axonal degeneration in vivo. J Neurosci. 2010;30(40):13291-304.[27]Beirowski B, Babetto E, Gilley J,et al. Non-nuclear Wld(S) determines its neuroprotective efficacy for axons and synapses in vivo. J Neurosci. 2009 ;29(3):653-668.[28]Conforti L, Gilley J,Coleman MP.Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci. 2014;15(6):394-409..[29]Felici R,Lapucci A, Ramazzotti M,et al. Insight into molecular and functional properties of NMNAT3 reveals new hints of NAD homeostasis within human mitochondria. PLoS One. 2013;8(10):e76938.[30]Yamamoto M, Hikosaka K, Mahmood A, et al.Nmnat3 Is Dispensable in Mitochondrial NAD Level Maintenance In Vivo. PLoS One. 2016;11(1):e0147037.[31]Conforti L, Janeckova L, Wagner D, et al. Reducing expression of DNA+ synthesizing enzyme Nmnat 1 does not affect the rate of Wallerian degeneration. FEBS J. 2011; 278(15): 2666-2679.[32]Zhai RG, Cao Y, Hiesinger PR,et al.Drosophila Nmnat maintains neural integrity independent of its DNA synthesis activity. PLoS biology. PLoS Biol. 2006;4(12):e416.[33]Verdin E. DNA + in aging, metabolism, and neurodegeneration. Science. 2015;350(6265):1208-1213.[34]Mack TG, Reiner M, Beirowski B,et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/ Nmnat chimeric gene.Nature neuroscience.2001;4(12):1199-206.[35]Gerdts J,Brace EJ, Sasaki Y,et al. Neurobiology. SARM1 activation triggers axon degeneration locally via DNA(+) destruction. Science. 2015;348:453-457.[36]Conforti L, Wilbrey A, Morreale G,et al. Wld S protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. J Cell Biol. 2009;184(4):491-500.[37]Milde S, Gilley J,Coleman MP.Subcellular localization determines the stability and axon protective capacity of axon survival factor Nmnat 2. PLoS Biol. 2013;11(4): e1001539.[38]Xiong X, Hao Y, Sun K, et al.The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein. PLoS Biol. 2012;10(12):e1001440.[39]Babetto E, Beirowski B, Russler EV, et al. The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction.Cell reports.2013;3:1422-1429.[40]Gilley J, Orsomando G, Nascimento-Ferreira I,et al. Absence of SARM1 rescues development and survival of Nmnat 2-deficient axons. Cell Rep. 2015;10(12):1974-1981.[41]Yang J, Wu Z, Renier N, et al.Pathological axonal death through a MAPK cascade that triggers a local energy deficit. Cell Cell. 2015;160(1-2):161-176.[42]Schlaepfer WW. Effects of energy deprivation on Wallerian degeneration in isolated segments of rat peripheral nerve. Brain research.1974;78:71-81.[43]Summers DW, DiAntonio A,Milbrandt J.Mitochondrial dysfunction induces Sarm1-dependent cell death in sensory neurons. J Neurosci. 2014;34(28):9338-9350.[44]Ikegami K,Koike T. Non-apoptotic neurite degeneration in apoptotic neuronal death: pivotal role of mitochondrial function in neurites. Neuroscience.2003;122, 617-626.[45]Alvarez S, Moldovan M,Krarup C. Acute energy restriction triggers Wallerian degeneration in mouse. Experimental neurology.2008;212:166-178.[46]Villegas R, Martinez NW, Lillo J, et al.Calcium release from intra-axonal endoplasmic reticulum leads to axon degeneration through mitochondrial dysfunction. J Neurosci. 2014 ;34(21):7179-7189.[47]Zala D, Hinckelmann MV, Yu H, et al.Vesicular glycolysis provides on-board energy for fast axonal transport.Cell. 2013;152(3):479-491. [48]Zheng J, Sun J, Lu X, et al.BDNF promotes the axonal regrowth after sciatic nerve crush through intrinsic neuronal capability upregulation and distal portion protection. Neurosci Lett. 2016;621:1-8.[49]Quan Q, Chang B, Meng HY, et al. Use of electrospinning to construct biomaterials for peripheral nerve regeneration. Rev Neurosci. 2016;27(7):761-768. .[50]Gu X, Ding F,Williams DF. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials.2014;35: 6143-6156.[51]Wang CY, Zhang KH, Fan CY,et al.Aligned natural-synthetic polyblend nanofibers for peripheral nerve regeneration. Acta Biomater. 2011;7(2):634-643.[52]Madduri S,Papaloizos M,Gander B.Trophically and topographically functionalized silk fibroin nerve conduits for guided peripheral nerve regeneration. Biomaterials.2009; 31:2323-2334.[53]Deister C,Schmidt CE.Optimizing neurotrophic factor combinations for neurite outgrowth. J Neural Eng. 2006; 3(2):172-179.[54]Cattin AL, Burden JJ, Van Emmenis L, et al.Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell.2015;162:1127-1139.[55]Greenberg DA,Jin K. From angiogenesis to neuropathology. Nature.2005;438: 954-959.[56]Madduri S, di Summa P, Papaloizos M, et al. Effect of controlled co-delivery of synergistic neurotrophic factors on early nerve regeneration in rats. Biomaterials.2010;31:8402-8409.[57]Daly W, Yao L, Zeugolis D, et al. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface. 2012;9(67):202-221.[58]McGrath AM, Novikova LN, Novikov LN,et al. BD PuraMatrix peptide hydrogel seeded with Schwann cells for peripheral nerve regeneration. Brain research bulletin.2010;83:207-213.[59]Oliveira JT, Almeida FM, Biancalana A, et al. Mesenchymal stem cells in a polycaprolactone conduit enhance median-nerve regeneration, prevent decrease of creatine phosphokinase levels in muscle, and improve functional recovery in mice. Neuroscience. 2010;170(4):1295-1303.[60]di Summa PG, Kingham PJ, Raffoul W, et al.Adipose-derived stem cells enhance peripheral nerve regeneration.J Plast Reconstr Aesthet Surg. 2010;63(9):1544-1552.[61]Khalifian S, Sarhane KA, Tammia M,et al.Stem cell-based approaches to improve nerve regeneration: potential implications for reconstructive transplantation? Arch Immunol Ther Exp (Warsz). 2015;63(1):15-30.[62]Ikeda M, Uemura T, Takamatsu K, et al.Acceleration of peripheral nerve regeneration using nerve conduits in combination with induced pluripotent stem cell technology and a basic fibroblast growth factor drug delivery system. J Biomed Mater Res A. 2014;102(5):1370-1378.[63]Gu X, Ding F,Yang Y,et al. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Progress in neurobiology.2011;93: 204-230.[64]Gu Y, Zhu J, Xue C,et al.Chitosan/silk fibroin-based, Schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps. Biomaterials. 2014;35(7):2253-2263.[65]Zhu S, Liu J, Zheng C, et al.Analysis of human acellular nerve allograft reconstruction of 64 injured nerves in the hand and upper extremity: a 3 year follow-up study. J Tissue Eng Regen Med. 2016 Apr 21..[66]Qiu T, Yin Y, Li B, Xie L, et al.PDLLA/PRGD/beta-TCP conduits build the neurotrophin-rich microenvironment suppressing the oxidative stress and promoting the sciatic nerve regeneration.J Biomed Mater Res A. 2014;102(10):3734-3743.[67]Xu X, Yee WC, Hwang PY, et al.Peripheral nerve regeneration with sustained release of poly(phosphoester) microencapsulated nerve growth factor within nerve guide conduits. Biomaterials. 2003;24(13):2405-2412.[68]Hobson MI.Increased vascularisation enhances axonal regeneration within an acellular nerve conduit. Ann R Coll Surg Engl. 2002;84(1):47-53.[69]Piquilloud G,Christen T,Pfister LA,et al.Variations in glial cell line-derived neurotrophic factor release from biodegradable nerve conduits modify the rate of functional motor recovery after rat primary nerve repairs. Eur J Neurosci. 2007 ;26(5): 1109-1117.[70]Wood MD, MacEwan MR, French AR, et al. Fibrin matrices with affinity-based delivery systems and neurotrophic factors promote functional nerve regeneration. Biotechnol Bioeng. 2010;106(6):970-979.[71]Shen H, Shen ZL, Zhang PH,et al.Ciliary neurotrophic factor-coated polylactic-polyglycolic acid chitosan nerve conduit promotes peripheral nerve regeneration in canine tibial nerve defect repair. J Biomed Mater Res B Appl Biomater. 2010;95(1):161-170.[72]Hernández-Cortés P, Toledo-Romero MA, Delgado M, et al.Peripheral nerve reconstruction with epsilon-caprolactone conduits seeded with vasoactive intestinal peptide gene-transfected mesenchymal stem cells in a rat model.J Neural Eng. 2014;11(4):046024. [73]Santiago LY,Clavijo-Alvarez J,Brayfield C,et al.Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transplant. 2009;18(2):145-58.[74]Ladak A, Olson J,Tredget EE,et al.Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model.Exp Neurol. 2011;228(2):242-252. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [3] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [4] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [5] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [6] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [9] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [10] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [11] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [12] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [13] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [14] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| [15] | Fang Xiaoyang, Tang Tian, Wang Nan, Qian Yuzhang, Xie Lin. Repair and regenerative therapies of the annulus fibrosus [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1582-1587. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||